Water Standard Enthalpy Of Formation . the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. The aggregate state is given in parentheses following the formula, such as: For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
from www.youtube.com
the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The aggregate state is given in parentheses following the formula, such as: the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. For example, although oxygen can exist as ozone (o 3 ), atomic. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation of any element in its standard state is zero by definition.
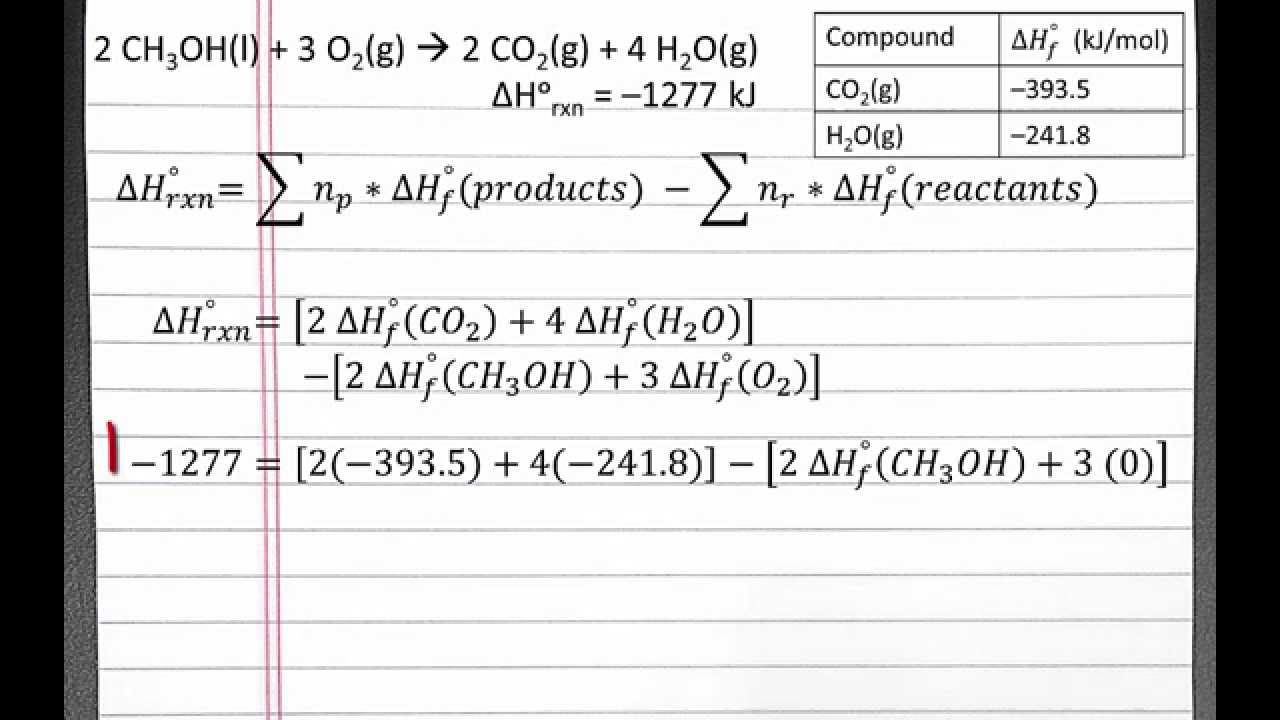
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube
Water Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. For example, although oxygen can exist as ozone (o 3 ), atomic. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The aggregate state is given in parentheses following the formula, such as:
From www.101diagrams.com
Enthalpy Diagram 101 Diagrams Water Standard Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation of any element in its standard. Water Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED Calculate the standard enthalpy of solution of AgBr(s) in water from the enthalpies of Water Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of any element in its standard state is zero by definition. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For. Water Standard Enthalpy Of Formation.
From pdfprof.com
enthalpies standard de formation et entropie standard Water Standard Enthalpy Of Formation the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. a standard enthalpy of formation $δh°_f$ is an enthalpy. Water Standard Enthalpy Of Formation.
From studylib.net
Standard Enthalpy of Formation Water Standard Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3 ), atomic. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. the standard. Water Standard Enthalpy Of Formation.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Water Standard Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3 ), atomic. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. 193 rows. Water Standard Enthalpy Of Formation.
From byjus.com
21. At 300 K standard enthalpy of formation of C6H5COOH(s), CO2(g), and H2O(l) are 408, 393, and Water Standard Enthalpy Of Formation the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is the enthalpy change when 1 mol of a. Water Standard Enthalpy Of Formation.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Water Standard Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its.. Water Standard Enthalpy Of Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Water Standard Enthalpy Of Formation the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. For example, although oxygen can exist as ozone (o 3 ), atomic. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure. Water Standard Enthalpy Of Formation.
From www.chemistryspace.com
Standard Enthalpy of Formation Water Standard Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3 ), atomic. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The aggregate state is given in. Water Standard Enthalpy Of Formation.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Water Standard Enthalpy Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. the standard enthalpy of formation, also known as the heat of formation, is the. Water Standard Enthalpy Of Formation.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Water Standard Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is. Water Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Water Standard Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. The aggregate state is given in parentheses following the formula,. Water Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED4 The standard enthalpy of formation of magnesium oxide_ Use Hess's Law and the following Water Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation, also. Water Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Water Standard Enthalpy Of Formation the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Water Standard Enthalpy Of Formation.
From studylib.net
2.5(a) Enthalpy Water Standard Enthalpy Of Formation The aggregate state is given in parentheses following the formula, such as: the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Water Standard Enthalpy Of Formation.
From dxorvtvqp.blob.core.windows.net
Standard Enthalpy Of Formation Gibbsite at Samuel Speed blog Water Standard Enthalpy Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3 ), atomic. . Water Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Molar Enthalpy PowerPoint Presentation, free download ID6624841 Water Standard Enthalpy Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. For example, although oxygen can exist as ozone (o 3 ), atomic. The aggregate state is given in parentheses following the formula, such as: the standard enthalpy of formation is a measure of the energy. Water Standard Enthalpy Of Formation.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Water Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Water Standard Enthalpy Of Formation.